Denis G. Rancourt, PhD
Summary
The cluster-randomized trial study of Abaluck et al. (2021) is fatally flawed, and therefore of no value for informing public health policy, for two main reasons:
These disjunctive fatal flaws are explained below. Either one is sufficient to invalidate the results and conclusions of Abaluck et al.
Furthermore, the Abaluck et al. symptomatic seroprevalence (SSP) results are prima facie statistically untenable. The treatment-to-control differences in numbers of symptomatic seropositive individuals are too small to rule out large unknown co-factor, baseline heterogeneity, and study-design bias effects. In addition, they are at best borderline significant, in terms of purely ideal-statistical estimations of uncertainty. Finally, the practice of using whole households while reporting on an individual basis, introduces unknown correlations/ clustering, and vitiates the mathematic assumptions that underlie the statistical method.
|
Table of Contents |
|
|
Purpose |
|
|
Summary |
|
|
Can the chosen antibody test be used in this application? |
|
|
|
Is the antibody assay specific for SARS-CoV-2? |
|
|
Is the antibody assay validated for use in Bangladesh? |
|
|
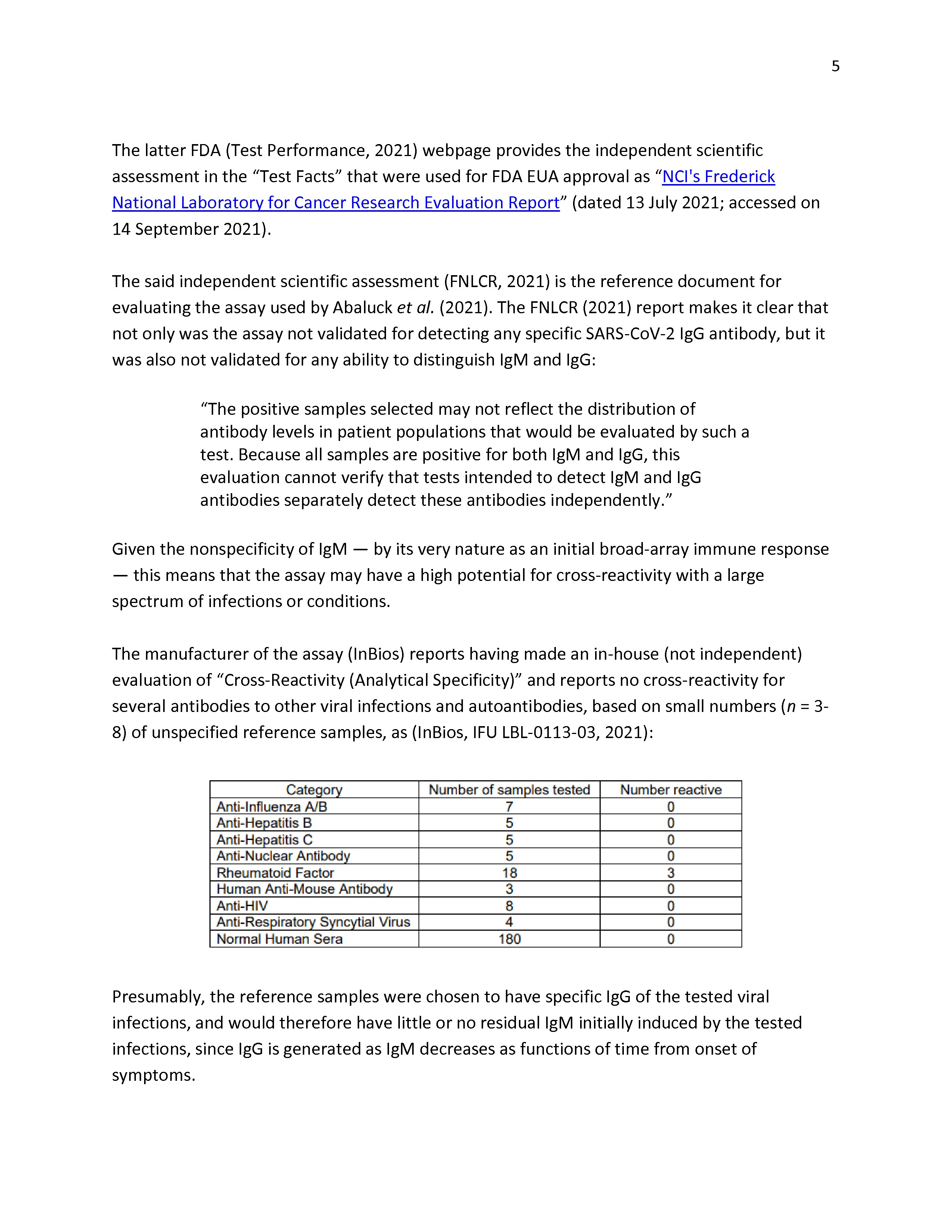
Was “spectrum bias” duly examined by InBios and Abaluck et al.? Are the positives reliable? |
|
|
Conclusion regarding the serology test |
|
Are the control and treatment arms valid (comparable)? |
|
|
|
Treatment alone versus adding super-treatment interventions |
|
|
Science of the stress-immune relationship |
|
|
Mechanisms of bias from the super-treatment interventions |
|
Is the size of the trial sufficient for the results to be reliable? |
|
|
|
All adults, 18 through 60+ years old, both mask types together |
|
|
Oldest age group, 60+ years old, surgical masks only |
|
Conclusion |
|
|
References |
|
|
Appendix A: Media reviews of the Abaluck et al. (2021) mask study |
|
|
My competence to review science about COVID-19 |
|