By Denis G. Rancourt, PhD
1 July 2023
The short answer is no.
The conclusion of Schmeling et al. [1]
“In conclusion, the results suggest the existence of a batch-dependent safety signal for the BNT162b2 vaccine, …”
does not follow from their analysis.
Let me explain why.
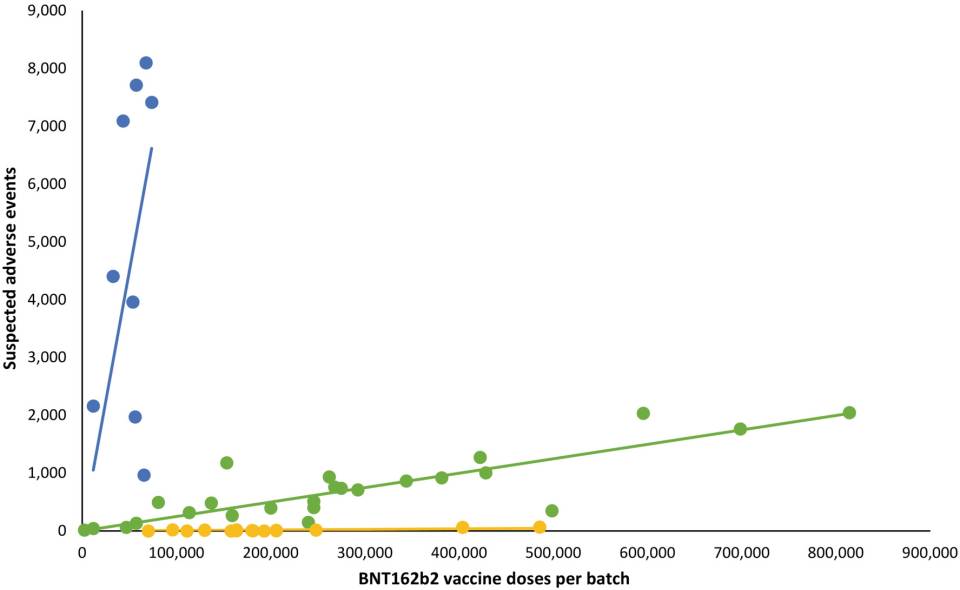
They assign reported adverse effects (AEs) to vaccine batches, and make and interpret graphs of number of batch-assigned AEs versus number of vaccine doses per batch, delivered in Denmark between 27 December 2020 and 11 January 2022. Each point on such a graph (for a given AE type or severity) is for one of the 52 batches used in Denmark in this period.
They show the resulting graph only for all-AEs, irrespective of type or severity (their Figure 1). They admit “Compared to the rates of all SAEs, serious SAEs and SAE-related deaths per 1000 doses were much less frequent and numbers of these SAEs per 1000 doses displayed considerably greater variability between batches, with lesser separation between the three trendlines,” but they do not show those graphs.
That is the full extent of their analysis. In doing this, they exclude all the information about the subjects experiencing the AEs, such as age and sex; and all the information about the injections, such as the date of the injection. They are blind to virtually everything except what is needed to make the batch assignment for each AE.
This blindness by design is acknowledged as: “in the present study, … , demographics of SAE cases, … , were not examined.”
This is a recipe for studying artifacts arising from invisible factors.
Specifically, here is a significant potential problem:
In fact, it has been shown ― both from a study of reported AEs in the USA (VAERS) [2] and from excess all-cause mortality synchronous with vaccine rollouts [3] ― that the risk of death following injection rises exponentially with age of the subject, doubling every 5-10 years in age.
This means that one must expect age to be a dominant factor, which causes large (order of magnitude) variability in deaths per dose, by age. This is crucial when injections are prioritised for the elderly population, and batches are used at different dates.
Hickey and Rancourt found (their Figure S6, [2]) that the age factor could explain all the batch differences in deaths, and that increased variability in deaths per dose was likewise consistent with increasing median age, in the USA (VAERS).
It was incorrect for Schmeling et al. to conclude as they did knowing that they had excluded the age consideration.
Of course, I do not mean that there are no manufacturing impurities in the vaccines, or that there cannot be “bad batches”, but I am not aware of any evidence that “bad batches” are a significant problem on population scales. The vaccines are toxic, as designed, and that is the main impact.
References
[1] Schmeling et al. (2023) “Batch-dependent safety of the BNT162b2 mRNA COVID-19 vaccine”. Eur J Clin Invest. 2023; https://doi.org/10.1111/eci.13998
[2] Hickey and Rancourt (2022) “Nature of the toxicity of the COVID-19 vaccines in the USA”. ResearchGate [Preprint] (9 February 2022); https://www.researchgate.net/publication/358489777_Nature_of_the_toxicity_of_the_COVID-19_vaccines_in_the_USA / Archived at: https://archive.ph/LZpRj
[3] Rancourt et al. (2023) “Age-stratified COVID-19 vaccine-dose fatality rate for Israel and Australia”. Correlation Research in the Public Interest, 9 February 2023; https://correlation-canada.org/report-age-stratified-covid-19-vaccine-dose-fatality-rate-for-israel-and-australia/

